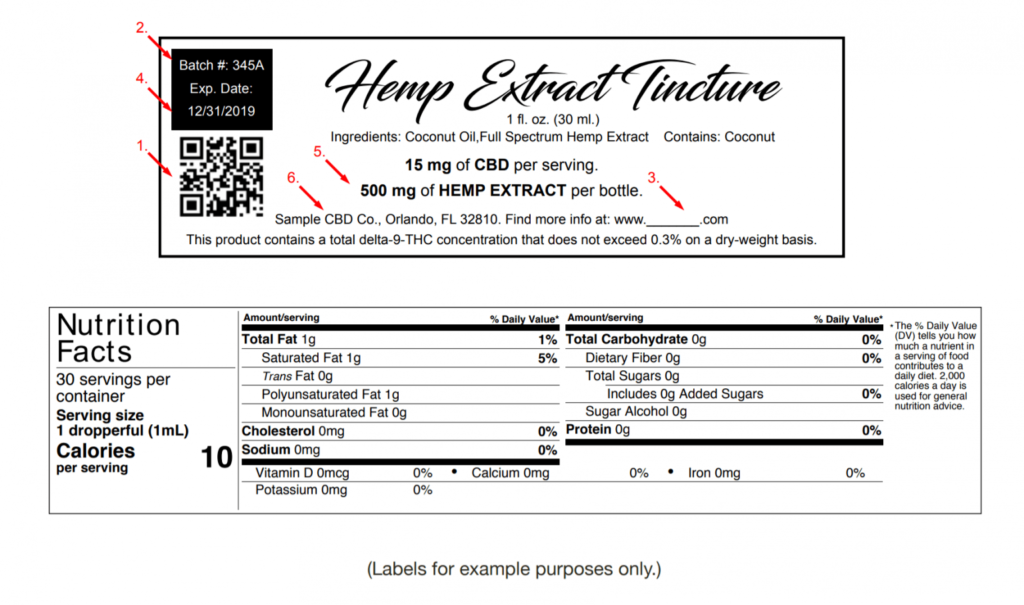
As of January 1, 2020, Florida’s new hemp extract guidelines went into effect. The biggest news was that a $650 yearly hemp extract license is required for any business in the state that manufactures, produces, or sells CBD products. In this article, we are covering their hemp extract labeling guidelines.
This is a requirement to sell your CBD products in stores in the state of Florida. So if you are a California or Texas brand this applies to you if you want to sell your products in the state of Florida.
Florida Hemp Extract labeling guidelines
Existing labeling requirements pursuant to 21 CFR Part 101 for all packaged food products include the following:
- The common name of the product.
- A list of ingredients (including sub-ingredients) in order of preponderance.
- The business name and address of the manufacturer, packer, or distributor.
- An accurate declaration of the quantity of the contents in proper dual units.
- A Nutrition Facts panel, unless exempt.
- The label, and advertisement, shall not contain claims indicating the product is intended for the diagnosis, cure, mitigation, treatment, or prevention of disease rendering it a drug as defined in 21 U.S.C. 321(g)(1)
In addition to the above requirements, food products containing hemp extract must be distributed and sold in packaging that includes:
- A scannable barcode or quick response code linked to the certificate of analysis of the hemp extract by an independent testing laboratory,
- The batch number,
- The internet address of a website where batch information may be obtained,
- The expiration date,
- The number of milligrams of hemp extract. The specific cannabinoids marketed must be listed. The serving size shall be displayed on the nutrition facts label of the product,
- A statement that the product contains a total delta-9 tetrahydrocannabinol concentration that does not exceed 0.3% on a dry-weight basis.